Real-time BOD monitoring is becoming increasingly popular, and it’s no surprise as the alternative is a 5 day wait for lab results. Continuous organics monitoring opens the door for many new treatment process control and optimization possibilities that can result in significant cost savings, improved water quality, and compliance peace of mind. Information can be utilized to more effectively dose coagulant chemicals to improve BOD and TSS removal efficiency at primary clarifiers or improve control of aeration rate and recycle ratio in activated sludge processes.
For treatment plants that are prone to organic loading spikes, such as industrial wastewater treatment plants, municipal wastewater plants receiving truck-delivered landfill leachate, or municipal wastewater plants receiving significant discharge from industrial facilities, rapid event detection is very beneficial for decision making and prevention of system failures.
A QUESTIONS WE GET ASKED OFTEN IS: HOW DO YOU MEASURE BOD, A TYPICALLY 5-DAY LAB TESTING, IN A REAL TIME?
In order to answer this question, we first need to realize that BOD, although a biochemical testing procedure where microorganisms’ oxygen consumption over 5 days or more is measured, is essentially an estimate of biodegradable organics in the water. Like other organics present in the water, biodegradable organics also absorb UV light, and therefore can be monitored by measuring the absorbance properties of water. This principle, known as spectrophotometry, also applies to COD and TOC where different test procedures than BOD are used but the end goal is still to estimate the level of organics in water. Spectrophotometry has traditionally been a lab measurement but now with technology advancements is available for the process environment. A follow-up question to this then would be: how does absorbance relate to organics?
Let’s start by reviewing what absorbance is.
WHAT IS ABSORBANCE?
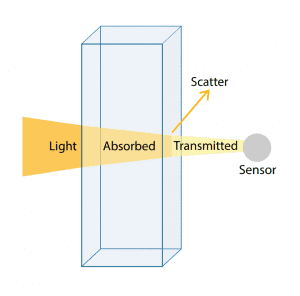 In simple terms, absorbance (or spectrophotometric) instruments pass light through a measurement cell containing a water sample and measure the amount of light that is transmitted across the sample column with a sensor on the other side of the cell. In practice, absorbance is calculated as a relative measure of the amount of light absorbed by a water sample compared with the amount of light absorbed by a pure water sample. The sample water measurement is divided by the pure water measurement before a logarithm is calculated. Therefore, any unit of measure of the light itself is cancelled in the division. The absorbance measurement results are expressed per path length, where typically per 1 cm or 1 m are used.
In simple terms, absorbance (or spectrophotometric) instruments pass light through a measurement cell containing a water sample and measure the amount of light that is transmitted across the sample column with a sensor on the other side of the cell. In practice, absorbance is calculated as a relative measure of the amount of light absorbed by a water sample compared with the amount of light absorbed by a pure water sample. The sample water measurement is divided by the pure water measurement before a logarithm is calculated. Therefore, any unit of measure of the light itself is cancelled in the division. The absorbance measurement results are expressed per path length, where typically per 1 cm or 1 m are used.
An example of an absorbance measurement would be 0.1 cm-1 or 10 m-1. Pure water (DI water) will read 0 cm-1, and completely opaque water will theoretically read infinity, due to the denominator becoming zero (no light making it through the sample cell). The inverse of absorbance is transmittance which instead provides a unit of measure of how well light is transmitted through water versus how much gets absorbed by compounds in water.
So the next question is what absorbs light in water?
WHAT ABSORBS LIGHT IN WATER?
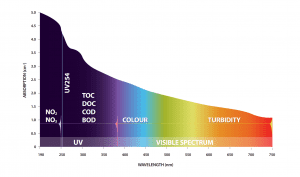
Many different compounds and substances absorb light including organic matter (BOD, COD, TOC, DOC, UV254), nitrate, nitrite, colour, colloidal solids, certain metals, pesticides, surfactants, diesel fuel and many others. Each substance has its own unique absorbance profile in the light spectrum, therefore if the profile is known then the appropriate wavelengths in the light spectrum can be used to measure that substance.
Industry experts, such as Real Tech, have built extensive libraries of absorbance profiles and offer spectral absorbance instruments that are dedicated to detecting and measuring many important parameters and contaminants of concern using this measurement principle.
Robust correlations or calibrations can be made to the water quality parameter or component desired to be monitored, such as BOD, thereby allowing for measurement utilizing spectral absorbance data.
Finally, let’s take a deeper look at how an absorbance measurement is translated into meaningful concentration information.
HOW DOES AN ABSORBANCE INSTRUMENT OUTPUT CONCENTRATION?
The relationship between absorbance (A) and concentration is defined by the Beer-Lambert Law (or Beer’s Law). Beer’s Law states that the absorbance of matter in water is directly proportional to its concentration, expressed by the following equation:
A = ε•b•c
Where ε is the molar absorptivity of the matter in the water sample, which is constant and specific for each compound, b is the thickness of the water sample column, and c is the concentration of matter in the water sample. Therefore, as an example, if the concentration of organic matter in the water were to double, then the absorbance would also double.
Because of this relationship, absorbance instrumentation can be programmed to output a concentration value, in mg/L or ppm, using a calibration. Most absorbance instruments come with a starter factory calibration for the parameters or compounds of choice, which can be further refined on-site to improve the accuracy and reliability if necessary.
To further simplify the use of absorbance instruments, some manufacturers, such as Real Tech, have created dedicated products for common water quality parameters such as BOD/COD, TOC, nitrate, nitrite, and others that have the wavelengths pre-selected and calibrations pre-programmed so the user can easily select a product that meets their detection demands.
Real Tech’s innovative technologies have allowed spectral absorbance (spectrophotometry) measuring techniques to move from the lab to the process environment in a simple and effective manner.
WHAT ARE THE BENEFITS OF USING SPECTRAL ABSORBANCE INSTRUMENTS FOR REAL-TIME WATER QUALITY MONITORING?
- The measurement process does not require sample preparation, nor does it require reagents or changing the sample composition in any way. Operation is very straight forward and maintaining the instrument is both easy and low cost.
- Spectral analysis is a multi-dimensional measurement, which allows multiple parameters to be measured with a single sensor.
- Measurement is very quick, completed in a matter of seconds in most cases, making spectral absorbance instruments an ideal tool for process control applications.
Real Tech’s solutions utilize the power of spectral analysis to provide practical, accurate and affordable real-time monitoring of BOD and many other important water quality parameters that are traditionally left to lab analysis, helping to advance the management of water and wastewater.
DOWNLOAD: FAQ – How we Measure BOD in Real Time – Real Tech