The purpose of source water monitoring is to enable drinking water treatment facilities to identify changes in water quality, implement treatment strategies based on the characteristics of the water, optimize the treatment processes, and take preventative actions to protect the source water from intentional and accidental contamination. Unexpected changes in water quality or the presence of atypical contaminants can adversely impact the ability of a treatment facility to meet its treatment goals and may put the public health at risk. As such, online monitoring of key water quality parameters plays a crucial role for achieving these goals by providing fast and reliable data that is spatially and temporally representative of the sample water. Although there are several possible locations for source water monitoring, it is most commonly done at the plant intake.
PARAMETERS FOR SOURCE WATER MONITORING AND THEIR IMPORTANCE FOR POTABLE WATER TREATMENT
UV254
UV254 is a simple to measure parameter that correlates well with DOC/TOC in most source waters. In addition to being a good surrogate for the concentration of organics in the water, UV254 is especially sensitive to the reactive portion of natural organic matter. This makes UV254 a more relevant parameter for evaluating the DBP formation potential resulting from the organic content of the water. As organics can control or significantly contribute to the charge neutralization and coagulation process, measuring UV254, in addition to turbidity, at the plant intake provides very valuable information for coagulation dose control. UV254 also relates well to organics that can be removed by carbon adsorption, and hence can be used as a tool to dose pace PAC or to determine efficiency and run-times for GAC systems.
DOC/TOC
The most common source of organics in source waters that are not influenced by human activities is natural organic matter from decaying plants and animals. Although not always a safety concern, these organic materials can cause aesthetic issues such as odour and colour in the water. Depending on the composition and level of organics present, the potential for disinfection by-products formation can significantly increase. DBPs have serious health implications, and therefore their concentrations are strictly regulated. Further, a high DOC/TOC may lead to biological growth in the distribution system triggering multiple adverse effects for the overall health of the distribution system and the public health. Since treatment processes where most DOC/TOC removal occurs are coagulation-flocculation-sedimentation and carbon adsorption, DOC/TOC monitoring would provide the highest value at plant intake as well as immediately after the treatment processes (e.g., after sedimentation, after GAC).
ALGAE
Algae can cause many problems for drinking water treatment operations ranging from poor sedimentation to filter clogging (short filter run times). In addition,
if the higher algae concentrations are accompanied by high algal toxin concentrations, an entire plant shut-down may be required to protect public health.
Currently, the water industry relies on laboratory analysis that can take 2 to 5 days to detect algae. Faster detection of rising algae concentrations can assist
with preventative measures and also provide an early warning system to better protect public health.
COLOUR
Colour in surface and ground waters results primarily from the presence of natural organic matter, particularly aquatic humic matter. Colour caused by humic matter typically has a yellow-brown hue. The spectrophotometric method recommended by Standard Methods for measuring colour requires detecting absorbance at a wavelength between 450 and 465 nm. However, because colour is typically caused by humic matter in surface waters an approximate measurement can also be made using absorbance at 254 nm. Industrial wastewaters can contain lignins, tannins, dyes, and other organic and inorganic chemicals that cause colour. Humic materials and the colour caused by these materials are removed from potable water supplies for aesthetic reasons
and for health reasons because they are precursors in the formation of disinfection by-products. True colour refers to colour of water from which turbidity
has been removed. Apparent colour includes colours due to suspended matter in addition to substances in solution. Colour measurements can provide
complementary information to UV254 and DOC/TOC readings. A sudden increase in colour may indicate industrial pollution.
NITRATE/NITRITE
Being highly water soluble, nitrates can migrate very fast in the water and reach different locations quickly. Fertilizers typically contain high concentrations of
nitrates, and agricultural, residential and commercial applications of fertilizers are major sources of nitrates in source waters. Other sources of nitrates are sewage disposal systems such as unmaintained septic tanks and leaking sewer pipes. It is important to monitor nitrates as often as possible since nitrate levels can be difficult to predict and may indicate presence of other contaminants. Nitrate is a regulated water quality parameter and the current USEPA maximum
contaminant level requires that nitrate concentration not exceed 10 mg/L as N. Smart water management requires reliable and quick detection of nitrates to
allow water utilities to take rapid action and protect public health against the hazards posed by nitrates. Online nitrate detection would particularly be
beneficial for treatment facilities withdrawing their waters from sources susceptible to agricultural and urban runoff.
SPECTRAL ABSORBANCE
As DOC/TOC is an aggregate measurement of organics in the water, often times no conclusive information can be derived on the composition of the organics.
Spectral absorbance brings a multidimensional approach to the table by allowing for the characterization of the organics in addition to their quantification. This is especially important in source waters that are under the influence of industrial and municipal wastewater discharges. The composition of organics helps identify the source of contamination along with determining the necessity to treat and applicable treatment technologies. Further, the DBP formation potential depends on the reactivity of the organics as much as it depends on the total quantity. Characterization of the source water at the intake would provide the most benefits by providing feedforward information to treatment processes.
pH
pH is a term used to define the intensity of a solution’s acid or alkaline condition. It is essentially a scale derived from the concentration of hydrogen ions in
the solution. It is a crucial factor affecting all aspects of water chemistry, and therefore plays an important role in many water treatment processes such as
coagulation, disinfection, chemical precipitation, corrosion control, and oxidation. pH monitoring at the plant intake can provide the additional benefit of
event detection as sudden changes in pH can indicate industrial discharge or accidental spills.
CONDUCTIVITY
The electrical conductivity of a solution is a measure of the ability to conduct electrical current. Because the electrical current is conducted via the ions in the solution, the conductivity increases as the concentration of ions do. This is why electrical conductivity is generally used as a surrogate measure for total dissolved solids concentration. Conductivity can be used to indicate industrial spill events in source water monitoring applications.
ORP
Oxidation reduction potential is a measure of the potential flow of electrons between oxidizers and reducers. This defines the oxidizing or reducing power
of a solution. As such, ORP indicates a water sample’s ability to break down contaminants. Changes in ORP of a water sample may indicate contamination by
industrial wastewater as large amounts of certain chemicals can greatly decrease or increase the ORP. ORP is also used in oxidation and disinfection processes as a process control tool.
DO
Most living organisms depend on oxygen in one form or another to maintain the metabolic processes that produce energy for growth and reproduction. Water solubility of oxygen is quite low and heavily depends on temperature varying from 14.6 mg/L at 0 °C to 7 mg/L at 35 °C at 1 atm pressure. The low solubility of oxygen is the primary reason why the purification capacity of natural waters is limited. Spikes in organics concentration in the water lead to reduced DO levels which can help identify industrial discharge or spills as well urban and agricultural runoff. In contrast, unusually high DO can indicate increased algal activity and can serve as an early warning for algae blooms and related problems.
TURBIDITY
Turbidity is caused by a wide variety of suspended materials that range in size from colloidal to coarse particles. In addition, materials contributing to turbidity range from purely inorganic substances to ones that are largely organic in nature. Turbidity is an important parameter for source water monitoring for three reasons: aesthetics, filterability and disinfection. Drinking water is expected to be turbidity free as the general public associates turbidity with contamination and pollution. Water filtration is negatively impacted by increasing turbidity levels as filter run times get shortened in addition to final quality of filtered water being decreased. Disinfection is another water treatment process that is negatively impacted by high turbidity. Effective disinfection requires full contact between the disinfecting agent and the target organisms. Turbidity may create a higher demand for the disinfectant, and in some cases fully or partially protecting the
microorganisms from coming into contact with the disinfectant, which results in incomplete disinfection. Turbidity is also used in determining the coagulant
dose necessary to meet process effluent targets.
EVENT DETECTION AND RELEVANT PARAMETERS
INDUSTRIAL DUMPING: pH, DOC/TOC, conductivity, DO, UV254, spectral absorbance, nitrate, colour
ACCIDENTAL SPILLS: DOC/TOC, conductivity, UV254, spectral absorbance, pH
ALGAL BLOOMS: Nitrate, algae, DO, turbidity
STORM RUNOFF: (URBAN AND RURAL) DOC/TOC, turbidity, spectral absorbance, UV254, conductivity, DO, nitrate
AGRICULTURAL RUNOFF: DOC/TOC, nitrite and nitrate, UV254, spectral absorbance, conductivity
DRINKING WATER TREATMENT PROCESSES THAT CAN BE OPTIMIZED BASED ON SOURCE WATER MONITORING
PRETREATMENT WITH PERMANGANATE AND PRE-OXIDATION
Manganese and iron can be detected via spectral absorption to help with dosing of permanganate. Alternatively, residual permanganate can be detected via absorbance readings to optimize dosing of permanganate. Best installation location: before or after filtration
COAGULATION/FLOCCULATION/SEDIMENTATION
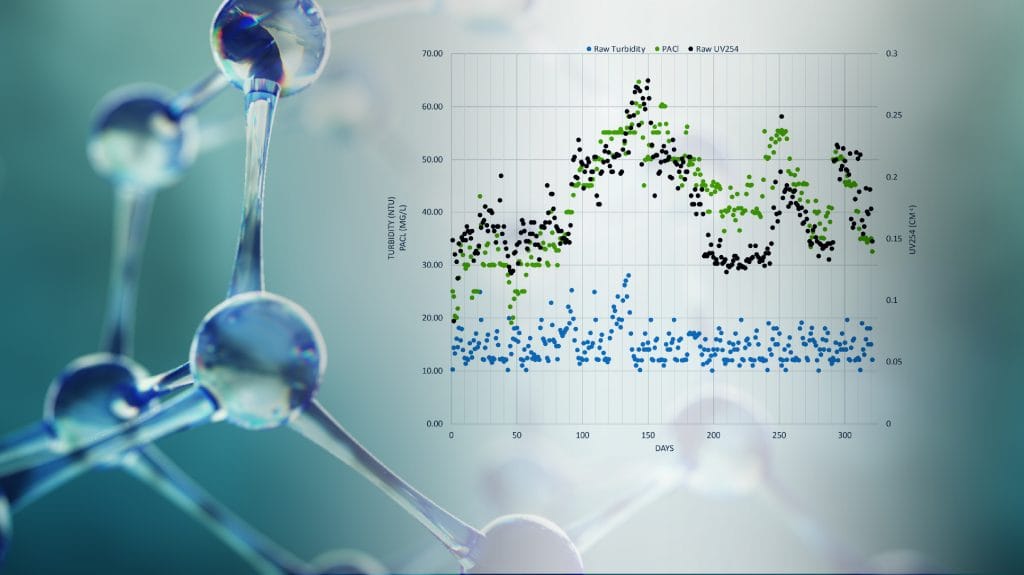
Effective removal of solids and organics during coagulation requires optimal dosing of coagulants, and in some cases also polymer coagulant aids and powdered activated carbon (PAC). The optimum dose depends on all water quality parameters that affect the water chemistry. Turbidity and UV254 are key parameters as they directly related to “how much” needs to be removed from the water. pH plays a key role as it controls the extent and speed of chemical reactions that take place between the coagulant and target colloidal materials. Operating the coagulation process effectively has significant benefits on the overall treatment plant performance: 1. Better organics removal yielding less DBP formation 2. Better filter run times 3. Better disinfection efficiency 4. Better quality water in the distribution system 5. Less organic loading and longer run time for the activated carbon process (if there is any).
PAC/GAC
UV254, TOC/DOC, spectral absorbance, algae Monitoring organics for dosing PAC or determining run-times for GAC. Ensuring performance.
DISINFECTION
DBP formation heavily depends on how much organics make it to the disinfection process. Furthermore, absorbance before and after disinfection is highly correlated to DBP concentrations. Both THMs and HAAs can be detected this way, which provides a better monitoring tool than online THM analyzers, which only monitor THMs.
DOWNLOAD ARTICLE PDF
________________________________________________________________________________________________________________________
RELATED POSTS
ORGANICS AREN’T INVISIBLE: A GUIDE FOR SIMPLE ONLINE MONITORING
CASE STUDY: SOURCE WATER MONITORING IMPROVES WTP PROCESS CONTROL
SOURCE WATER MONITORING: ORGANICS OR COLOUR
GO BACK TO BLOG
![]()