UV ABSORBANCE
The UV absorbance is calculated as a relative measure of the amount of light absorbed by a water sample compared with the amount of light absorbed by a pure water sample. The sample water measurement is actually divided by the pure water measurement before a logarithm is calculated. Therefore, any unit of measure of the light itself is cancelled in the division. An example of an absorbance measurement would be 0.1 A/cm. Pure water (DI water) will read 0.0 A, and completely opaque water will theoretically read infinity A, due to the logarithm.
UVA AND CONCENTRATION
UVA is directly proportional to the concentration of matter in the water. UVA is given by the following equation: UVA = ε•b•c where ε is the molar absorbtivity of the particular type of matter in the water sample, b is the path length of the water sample, and c is the concentration of matter in the water sample. Therefore, as an example, if the concentration of organic material in the water were to double, then the UVA would also double.
USING UVA TO MONITOR WATER QUALITY PARAMETERS AND COMPOUNDS
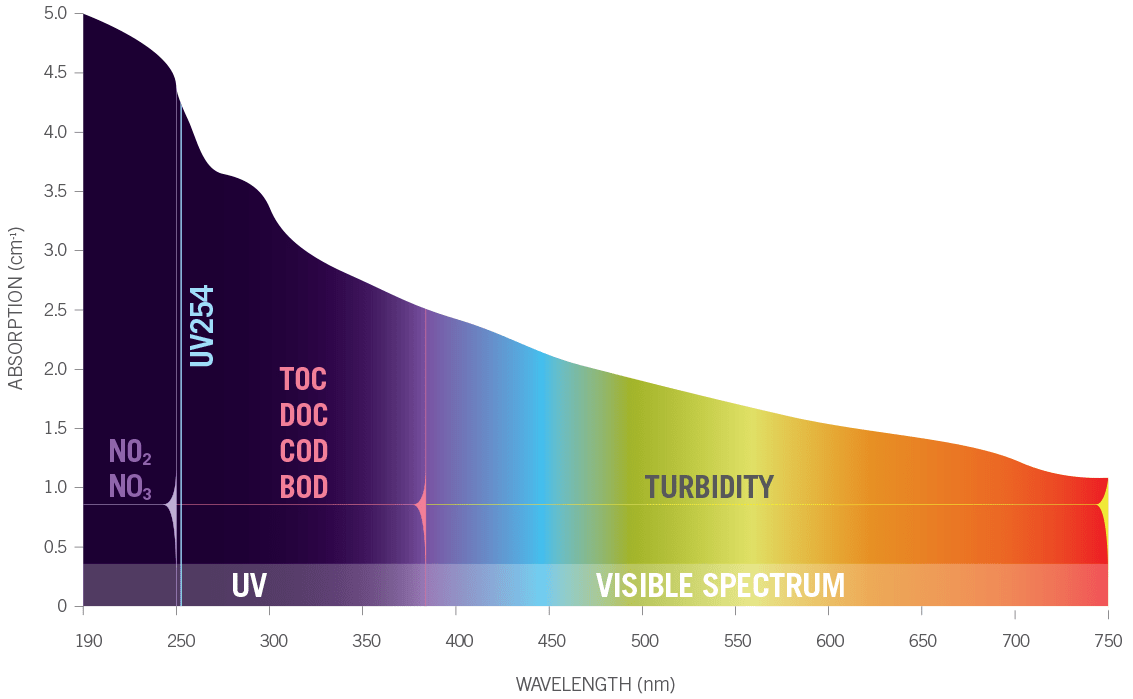
Many different substances absorb and scatter UV light including: organic matter, nitrates, nitrites, colloidal solids, certain metals, pesticides, diesel fuel and more. Different substances absorb at certain wavelengths of UV light more than others. Some substances such as colloidal solids tend to scatter UV light more than they absorb it. Knowing how different substances absorb or scatter UV light allows Real Tech to measure the levels of these different substances in the water.
TYPES OF UVA INSTRUMENTS
UVA instruments range from single wavelength up to full spectrum. Selection of an instrument depends on the parameters of interest and application. Single wavelength instruments are commonly used for parameters such as UV254 (organics) or UVT, colour or TSS. In more complex waters, multiple wavelength instruments are favored for detection of one or more parameters, such as nitrate, nitrite, algae, BOD, COD, TOC etc., where additional reference wavelengths are used to provide compensation from interfering compounds. Full spectrum instrumentation provides detection for the most complex applications, monitoring many difficult to detect compounds as well as differentiation of parameters that absorbance within the same wavelength range, such as nitrate (NO3) and nitrite (NO2).
GO BACK TO BLOG
![]()